 By Julie Beal
By Julie Beal
Having explained what lipid nanoparticles are, and how they work, in Part 1 and Part 2, this article will move on to examine the nature of the corona and the involvement of PEG, and the fancy routines they’re meant to perform. Together with previous articles, this helps define the context in which the genetic vaccines came into being, and exposes the narrative of ‘newness’ as a disingenuous sham.
The Protein-Corona
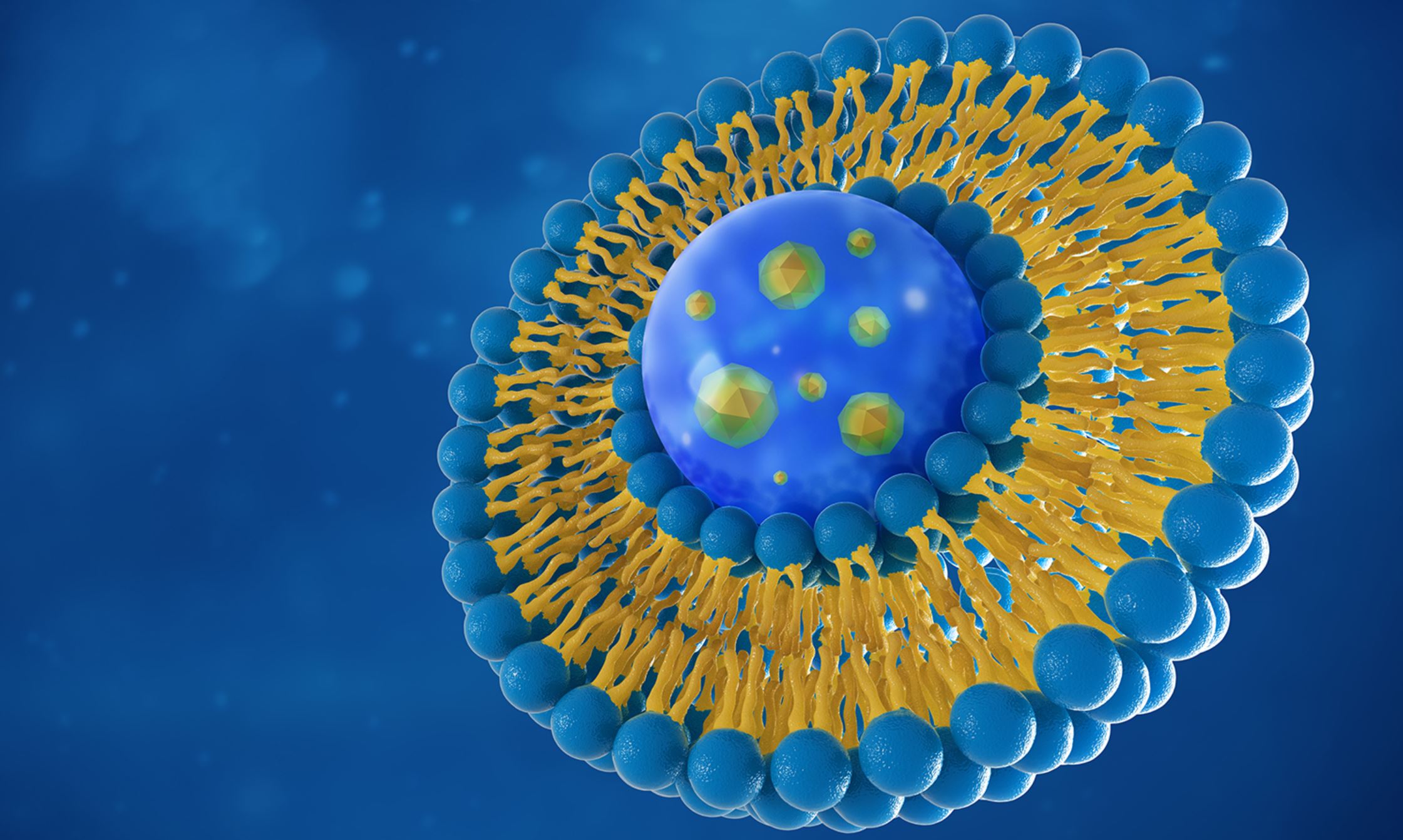
As soon as nanoparticles are injected into the body, proteins start sticking to them, forming a layer called the corona (which is Latin for ‘crown’). The corona gives each nanoparticle a whole new biological identity that’s very different to the one designed by the manufacturer, and changes the way the body reacts. It goes from being a synthetic, chemical thing, to an actively biological thing, and it keeps changing as it travels round the body, in what’s known as ‘the Vroman effect’. The proteins in the corona interact with the lipids in the nanoparticle, exchanging components, changing shape, and continually morphing into something new. This process involves “a dynamic interaction between NPs and biomolecular species and other chemical and organic matter”. The corona can affect how stable the LNPs are, where they end up, and the way the immune system responds to them; and all of this depends on which proteins get attached, and what the nanoparticle is made of. However, a lack of research means the nanoparticle-corona “is still not well understood”. Many researchers agree, however, that understanding the corona formation process, and the types of protein involved, “is crucial in predicting NP behavior in biological systems”. Playing a significant role in all of this is PEG because of its link to the corona, and the induction of autoimmune disorders.
PEG is a stealth polymer
PEG (short for polyethylene glycol) is a polymer that’s used in all sorts of things, from spandex and laxatives, to toothpaste and beauty products. A polymer is something that’s made up of lots of monomers, or molecules (poly = multiple; mer = units), so a polymer that’s made by bonding styrene monomer molecules together is called polystyrene. PEG is added to the mRNA vaccines to stop the NPs sticking to each other, and in order to, “shield the nanoparticles from recognition by the immune system”, i.e., it prevents the NPs from being seen by the immune system, and then destroyed by various immune cells, such as macrophages. Because of this, it’s considered to be “the most widely used ‘stealth’ polymer in the drug delivery field”. PEG creates a hydrophilic polymer coating which acts as a ‘steric barrier’ that stops proteins sticking to it because proteins tend to stick to things that are hydrophobic (i.e. they repel water). This means the NPs manage to avoid interactions as part of the stealth effect, which means they have a “higher circulation time and lower elimination rate”, i.e. they stay in the body for longer, and reach more places. However, it doesn’t last long, because the PEG is designed to come away, or dissociate, from the LNP shortly after being injected.
The fate of PEG
At first, the PEG helps the LNPs to get away from the site of injection, so they can get into the bloodstream and spread around the body. PEG doesn’t totally prevent stuff sticking to the LNPs, but it does make the LNPs fairly ‘unrecognizable’, and if they didn’t have PEG on them, the body could react by dealing with them as ‘foreign objects’. A hard lump would form where the needle went in, and the LNPs could then be surrounded and destroyed by immune cells. When PEG is attached to a special kind of lipid (with short acyl chains), it can come away from the LNP after about an hour. With other lipids, it might take a few days to shed the PEG. The loss of PEG initiates a new stage in the life-cycle of the LNPs, as they now begin to fully interact with proteins in the blood:
“LNPs are known to interact with serum proteins, exchanging components and acquiring proteins in circulation that can potentially direct LNPs to specific cell types.”
By this point, the LNP has become hydrophobic. This is why proteins can bind to it as part of the corona, and is the only way the LNP can get into a cell. But what happens to the PEG while it’s on the nanoparticle, and what happens after it’s come off? There’s little discussion about this, because PEG is classed as non-toxic and non-biodegradable, so its fate is said to be simple – it’s supposed to just pass out of the body, without having an effect on it, because humans don’t have the right kind of enzymes to metabolize PEG. In fact, there’s very little research on the fate of PEG at the cellular level, although there is some evidence that PEG can get broken down in the body.
Although PEG is considered to be biologically inert, experience with PEGylated hydrogel implants suggests otherwise, and some researchers claim it can be degraded inside cells by ROS, which can have biotoxic effects on the body. Degradation of PEG can create metabolites or “products that are toxic to the cells and tissues”, and it seems it’s possible for inflammatory responses to initiate the degradation of PEG by oxidising it. Oxidation is the loss of electrons, and it’s what happens to PEG found in waste-water systems, and on crops; specific kinds of bacteria are able to metabolize and degrade the PEG, and the first thing they do is oxidise it. Oxidation can also take place in the human body when special immune cells involved in inflammation produce reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). Both ROS and RNS are toxic, and can cause oxidative stress, and there is said to be “overwhelming evidence that the over-production of ROS is the major cause of the biotoxicity” of nanoparticles in general.
Knock, knock, who’s there?
LNPs can’t get into cells by themselves – they actually need a protein to bind to them. The protein acts as a transporter and a key, by taking the LNP to a cell receptor which lets it in because it looks like the right kind of protein. It’s kind of like on the guest list. The trouble is, it’s impossible to know which type of cells the LNPs will end up in, because there are more than 2000 proteins in blood-plasma, and at least 125 of them have been found in nanoparticle-coronas, especially apolipoproteins. For instance, the top 15 proteins found in coronas that formed on silica nanoparticles included several types of apolipoprotein, histidine-rich glycoprotein, serum amyloid A-4 protein, transthyretin, serum albumin, and immunoglobulin kappa constant. It’s also possible for complement proteins to become part of the corona: “In many reports, the protein corona formed on metal, lipid, and polymer nanoparticles appears generally composed of complement proteins and apolipoproteins with or without immunoglobulins.” The presence of complement proteins raises a red flag because they’re linked to hypersensitive reactions (discussed below).
All of this means there are lots of possibilities in terms of what can form a corona, and each one can have different effects. But it seems many of the LNPs in the mRNA coronavirus vaccines may actually end up in the liver, because they share many of the same properties as the LNPs contained in a nano-drug called patisiran. There are three references to patisiran in the EMA/Pfizer report, e.g. as evidence of the half-life of some of the lipids. Patisiran is a RNA gene-therapy product designed to treat transthyretin amyloidosis, a disease in which people produce mis-folded proteins in the liver. Patisran targets the TTR gene, and uses LNPs to deliver a kind of RNA called small interfering ribonucleic acid, or siRNA, to ‘silence’ the gene, and the LNPs tend to bind to Apolipoprotein E (ApoE).
ApoE interacts with natural lipid particles during “the trafficking of lipids in the bloodstream”. It’s mainly synthesized in the liver, and is a type of low-density lipoprotein (LDL) that can “remove excess cholesterol from the blood and carry it to the liver for processing.” Apparently, “lipid nanoparticles can naturally target the liver” because ApoE tends to bind to them, then gets them into liver cells using the LDL receptor. This begins when the PEG-lipids on the surface of LNPs are exchanged for proteins (such as ApoE). For instance, according to the EMA/Pfizer report:
“PEGylated lipid can exchange out of the LNP after administration, thus allowing the desired binding of endogenous proteins (e.g. Apolipoprotein E) and removing the steric barrier that would otherwise restrict interactions of the LNP with target cells and proteins.”
The EMA/Moderna report doesn’t mention ApoE, but it’s possible their LNPs also hitch a ride on it. This was suggested by a research article they published in 2018, in which they described testing their LNPs on mice. The tests revealed the LNPs didn’t work on mice who’d had specific genes removed i.e. the genes for ApoE and LDL. This suggests they didn’t have the ‘right kind of proteins’ available for the LNPs to bind to. All of this is worth explaining some more, not as a lesson on biology, but because, i) it’s part of the timeline leading up to the ronascam, ii) it’s an example of the level of detail the manufacturers have gone to, iii) it suggests a link to the liver, and, iv) it highlights some of the differences between the LNPs in the Pfizer and Moderna vaccines.
Patisiran
Sold with the brand name ONPATTRO, patisiran was licensed by the FDA in 2018, only to be sold with a largely unaffordable price-tag of around $400,000 per person per year (although the price for RNA therapeutics will probably decline to marketable levels if the factories can be kept going with the production of vaccines).
“In 2018, patisiran broke away from the pack to win regulatory approval on the strength of a pivotal trial that showed it had a good safety profile and led to meaningful improvement in the health and quality of life of people with hereditary transthyretin amyloidosis. The decision vindicated researchers who had stuck by RNAi, and particularly those at Alnylam.”
The biotech industry was buoyed by this development because, “…. it shows investors that there are markets that one can go after with this technology where there is a likely return on investment.”
The basic four-lipid recipe that was licensed for patisiran is the one being used by both Pfizer and Moderna, i.e., an ionizable lipid, a PEGylated lipid, DSPC, and cholesterol. But each company is using proprietary blends, which also means they can be patented.
Lipid Engineering
The ionizable amino‐lipid in the mRNA LNPs is designed to do a special trick. It starts off with a neutral charge (i.e., it’s neither positive, nor negative), but it changes after being taken up by a cell (into the endosome). The acidic pH of the endosome causes a reaction with the lipid that ionizes it so that it has a positive charge (i.e., it becomes cationic). Having a positive charge at this point helps get the LNP out of the endosome and into the cytoplasm of the cell, where the mRNA gets translated (this is where the body makes the spike protein by following the genetic instructions).
The amino-lipid used in the Pfizer vaccine is called ALC-0315 and is said to be similar to the amino-lipid in patisiran, which is called DLin-MC3-DMA, or just ‘MC3’. The EMA/Pfizer report draws parallels with patisiran, e.g., in reference to the half-life of the amino-lipid, which is expected to be approximately “20-30 days in human for ALC-0315 and 4-5 months for 95% elimination of the lipid”. The PEGylated lipid (ALC-0159) has been designed “to largely exchange out of the LNP after administration and before uptake into target cells”, whereas ALC-0315 “must remain with the LNP”, so that it can transition through the endosome.
Experiments have indicated that “the limiting step for protein production is release of the mRNA from endosomes (endosomal escape) and that this process per se is critically dependent on LNP particle size and cell type”. It’s also been shown that “small structural variations” in the amino-lipid can help with this, i.e., more of the mRNA manages to escape the endosome, so more proteins get translated, meaning there are also more antibodies made (ker-ching!). The amino-lipid that had to be bettered was MC3, and this is what Moderna claim to have achieved with one they developed a few years ago, so it might be the one they’re using in their ronavax, called ‘SM-102’.
In 2016, Moderna had a patent for a RSV vaccine made using MC3, and they were still using it in 2017 when they published results of tests they’d done on rats and monkeys (this report featured eight authors from Moderna, plus one from each of the other organisations, which were AstraZeneca, PureTech Health, Akcea Therapeutics, and Alnylam (the manufacturers of Onpattro). By 2018, however, Moderna had come up with their improved version of MC3, which they claimed had “increased endosomal escape” and fivefold higher mRNA expression compared to MC3. In their report, Moderna said that ApoE is known to quickly bind to MC3-based LNPs, which enables them to be taken up into liver cells by the low-density lipoprotein receptor (as described earlier!). They also pointed out that MC3 has a long half-life in the body, and that it could cause feisty immune reactions, and damage the liver: “LNP-related toxicities associated with MC3-based LNP systems are generally associated with immunological (cytokine and complement activation) and hepatic injury.” In 2019, Moderna published details of LNPs which they’d prepared using their new improved formula, and a technique called “ethanol drop nanoprecipitation”. This is the same technique they’re using for their ronavax, so, yes, Moderna have been working on these things for a while.
In terms of how long Moderna’s amino-lipid stays in the body, the EMA/Moderna report doesn’t mention half-life. Moderna say they’ve only got data for ‘SM-86’, (i.e., “a close structural analogue” to SM-102), which they say is ‘rapidly’ eliminated “via biliary and renal clearance” (meaning it goes through the biliary tract, and the kidneys). Other tests done on SM-86 “revealed no persistence of the lipid component in any tissue beyond 168 hours” (which is seven days).
Anti-drug antibodies
For many years now, it’s been known that people can develop anti-drug antibodies (ADAs) in reaction to some component of a drug they receive. Then, if they get given the drug again, they make antibodies that destroy it; for instance, there are reports of anti-DPPC antibodies (specific for phosphocholine) being induced by DPPC-liposomes in a vaccine. Another possibility is the induction of antiphospholipid antibodies, which is linked to Antiphospholipid Antibody Syndrome (APS) and can involve antibodies against cardiolipin, or a special type of glycoprotein. APS may develop as a result of molecular mimicry. This is when viruses or bacteria (or their synthetic analogues) have similar molecular patterns to human proteins, leading to the production of antibodies that target both. For instance, researchers have found “evidence that links the development of APS with exposure to microbial antigens, either during infection or vaccination.” It therefore seems feasible that people could form antibodies against some of the lipid or genetic components used in the mRNA LNPs, as well as PEG.
Anti-PEG antibodies
“PEG has never been used before in an approved vaccine, but it is found in many drugs that have occasionally triggered anaphylaxis—a potentially life-threatening reaction that can cause rashes, a plummeting blood pressure, shortness of breath, and a fast heartbeat.”
The PEG in the mRNA coronavirus vaccines has been linked to anaphylaxis, as have a number of so-called “nanomedicines” that also contain PEG. These PEGylated nano-drugs are usually given intravenously to very sick people, and are associated with various hypersensitivity reactions (HSRs). They are probably the reason why the link to the LNPs in the vax has been acknowledged. As well as anaphylaxis, HSRs can involve shortness of breath, facial redness and swelling, chest pain, chills, rash, and fever; they’re classed as ‘pseudoallergic’ reactions, because they result from the activation of complement. More than 30 proteins make up the complement system, and once activated, they set off a cascade of reactions, one of which is the release of anaphylatoxins, which then cause the release of histamine. When this system goes too far, it can cause dangerous inflammatory reactions, significant tissue damage and heart problems.
There is evidence to suggest that pre-existing anti-PEG antibodies can activate complement, both of which are linked to PEGylated nanomedicines and HSRs. Some of these drugs use lipid bubbles as a delivery vehicle; for instance, a cancer treatment called Doxil uses PEGylated liposomes that are similar to the PEGylated lipid nanoparticles in the mRNA vaccines. Doxil was found to cause severe hypersensitivity reactions in 25% of patients and complement activation in 72% of patients, whilst another study detected anti-PEG antibodies in 37% of patients after just one dose. Lots of healthy people produce anti-PEG antibodies, which can also have harmful effects, and also shows that PEG can be degraded in the body. Drug manufacturers have paid a lot of attention to this issue because anti-drug antibodies wage a battle in the blood that literally destroys their product. Anaphylaxis is observable, happens pretty much straight away, and can be easily related to the product. Nonetheless, the PEG in the vaccines is considered to be ‘safe’ because the doses are smaller and fewer.
The effect of PEG in patisiran (ONPATTRO) was assessed “by measuring antibodies specific to PEG2000-C-DMG, a lipid component exposed on the surface of ONPATTRO”. These tests involved using blood serum from “bioreclamation”, and Anti-PEG methoxy group Rabbit Monoclonal Antibodies, spiked with PEG2000-C–DMG. According to the FDA, the results were acceptable because PEG isn’t a natural human protein; “The ADA are to PEG, which is not endogenous to humans and so there is low risk of patients developing deficiency syndromes.”
Things that make you go ‘Hmm…’
As described in Part 2 of this article, various impurities, such as ‘lipid-RNA species’, have been found in both of the mRNA vaccines. Some of the ones in the Pfizer vax have apparently been translated into proteins, so they’ve been asked by the EMA to see if any of them have parts that are similar to human proteins, due to concerns about molecular mimicry that could cause an autoimmune disorder. But what about the potential for molecular mimicry with other components in the vaccines? What about the lipids, the dsRNA, and the spike protein itself? Some researchers have looked for similarities between parts of the rona, and parts of human proteins, to see if molecular mimicry might occur. Lots of similarities have been found, such as these three peptides: HVNNS, GIGVT, NESLI (the letters indicate an amino acid). These peptides are all found in the spike of the rona, as well as in a human protein involved in lipid metabolism and ApoE receptor binding. (The NESLI peptide is also found in Pneumocystis carinii.) The mRNA vaccines both contain a modified version of the genetic sequence for the spike protein that was published by China on the 17th of January, 2020. The potential for molecular mimicry comes from peptides that enter the body, either as a result of an infection, or from a vaccine.
Another thing to make you go ‘Hmm’ is the question of the role of ApoE. Is it also possible, for instance, that people could be at risk in terms of their genetic make-up? For example, people who’ve got the Apolipoprotein E4 allele can be genetically susceptible to Alzheimer’s Disease. And what might happen if a LNP reached the brain? ApoE actively transports cholesterol between neurons, e.g., to help with cell repair, so could LNPs cause other neurological effects in connection with this?
Why haven’t Moderna, or any of the other companies, shared data from previous trials? Is there something to hide? And why didn’t the EMA mention the unnatural sequences the vaccines contain? Strictly speaking, it’s not just ‘mRNA’ they contain, but modified mRNA, and is part of a ‘pick and mix’ approach to genetics that could threaten the human race.
Read Part 1
Read Part 2
Image credit: Wyatt Technology
Become a Patron!
Or support us at SubscribeStar
Donate cryptocurrency HERE
Subscribe to Activist Post for truth, peace, and freedom news. Follow us on Telegram, SoMee, HIVE, Flote, Minds, MeWe, Twitter, Gab and Ruqqus.
Provide, Protect and Profit from what’s coming! Get a free issue of Counter Markets today.

Be the first to comment on "Ronavax Roulette: Lipid Nanoparticles — PEG And The Protein-Corona (Part Three)"