 By Julie Beal
By Julie Beal
A number of coronavirus vaccines are formulated with lipid nanoparticles, and concerns have been raised over possible reactions to the PEG they contain, since anti-PEG antibodies can cause anaphylaxis. But did you know that nanoparticles can get into the brain? And that proteins can get stuck to nanoparticles, and cause autoimmune disease? PEG is added to try to prevent this from happening, but what on earth is PEG? And for that matter, what on earth is a lipid? Or a nanoparticle?
The second part of this article will take a closer look at some of these issues, but will begin here by looking at some of the basics, such as what lipids are, and the potential toxicity of lipid nanoparticles (LNPs). It’ll also provide some examples of how lipid metabolism is linked to a variety of disorders, and even immune reactions; this illustrates the importance of lipids in our cells, and raises a number of questions. Could LNPs affect neurons? Could they damage cells, and trigger a disorder of some kind? So far, studies of LNPs seem limited to the lab, so these potential effects on humans may not have been investigated.
What is a lipid?
“A lipid is any of various organic compounds that are insoluble in water. They include fats, waxes, oils, hormones, and certain components of membranes and function as energy-storage molecules and chemical messengers.” All cells are covered with membranes that contain proteins and lipids. There are also membranes around some bits inside the cell, such as the nucleus. These membranes can contain a staggering variety of lipids; there can be “as many as 10,000 to 100,000 different lipid species in a cell”, and they’re involved in regulating lots of different functions, such as signalling to other cells.
We’ve got four main types of phospholipids in our cell membranes, as well as various glycolipids, and cholesterol. The types of lipid the cell contains depends on what kind of cell it is, and the job it’s doing. But it’s really important to have the right mix of lipids, or things can go wrong. Whatever mix is required, the lipids have to be in the right proportions, so it’s something the body seeks to control very carefully. When there’s an imbalance, it can affect a range of different physiological processes, and is seen in a lot of diseases.
The membrane is there to provide protection, and is made of lipid bilayers, which allows it to retain fluidity, e.g. it can “freely rotate and move in lateral directions”. The level of fluidity in the cell membrane is partly determined by temperature, but also by the type of lipid-mix, and cholesterol is an important part of this.
Viruses and bacteria are also coated in lipids, and this plays a key role in how some of the lipid adjuvants operate, because we’ve got cells in our bodies that can recognize some of the lipid molecules on a range of pathogens.
What is a lipid nanoparticle?
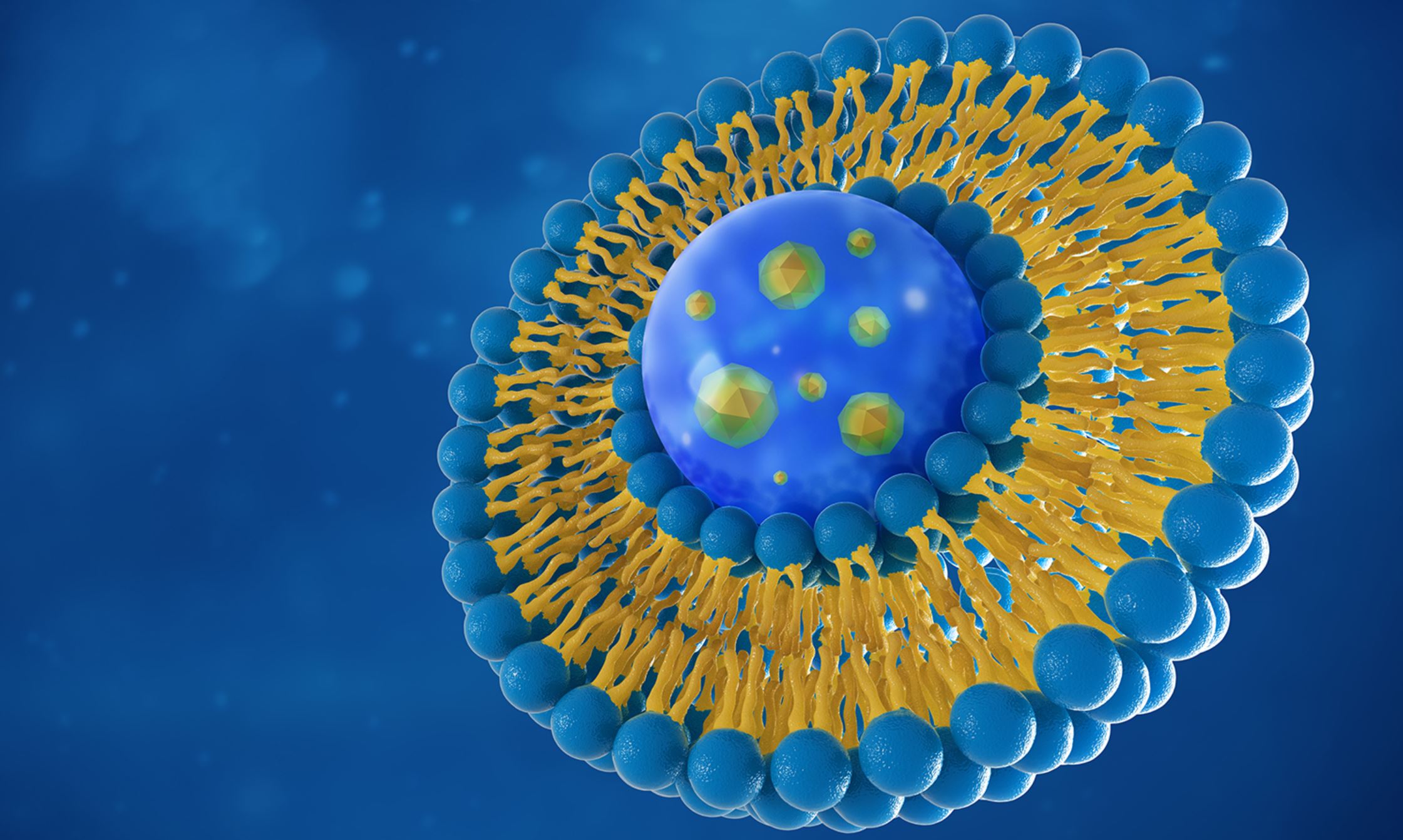
According to Precision Nanosystems, “Lipid nanoparticles (LNPs) are the most clinically advanced non-viral gene delivery system. Lipid nanoparticles safely and effectively deliver nucleic acids, overcoming a major barrier preventing the development and use of genetic medicines.”
The desire to use mRNA is strong, partly because there are plans to use it to treat “undruggable diseases”, but genetic constructs such as mRNA are macromolecules that can’t get through the cell membrane, because they’re way too big. They also get degraded by immune responses en route to the cell, so they have to be hidden inside something. There are very particular requirements for this job, and LNPs are said to fit the bill.
LNPs are similar to many human cells, in that they are liquid crystalline structures. A variety of lipids (e.g. oils and fats) can be synthesized and/or processed to produce nano-sized structures. When the lipids are subjected to processes such as microfluidics, they undergo phase transitions between various structural states, and they can be made into particular shapes, depending on which part of the phase transition they’re in. It’s possible to exploit these transitions to make them into certain shapes, such as rods, cubes, spheres, or hexagons.
LNPs have a similar design to our cells, in the sense that they are watery blobs with a lipid membrane that’s got two layers, so it’s called a ‘bilayer’. When a cell and a nanoparticle meet, they can fuse together if conditions are just right. When fusion occurs, a ‘stalk’ forms between the two blobs, creating a connection between their watery insides, and the contents of the LNP can pass through this connecting stalk, into the cell. The lipids are designed to undergo a phase transition; it’s triggered by a change in pH when they enter the cell. This allows the mRNA to be released and ‘translated’, (for example, the mRNA is processed to produce a coronavirus protein).
Although LNPs are used to shield genetic constructs until they reach the cell, the LNPs themselves also need to be shielded from being degraded by enzymes in the blood, or being attacked by the immune system. PEG (which is short for polyethylene glycol) is a polymer that’s added to the surface of some lipid nanoparticles to form a barrier, to avoid being recognized by the immune system.
Several properties of LNPs can be manipulated to achieve different effects, such as the degree to which they disrupt cell membranes. Also, because they stimulate the immune system, and are classed as an adjuvant, they can be designed to produce a particular kind of immune response. For example, “LNPs containing cationic lipids … induce a strong proinflammatory response with Th1 type cytokines” (a Th1-type response is one of the main targets for coronavirus vaccines).
Nanoparticle Toxicity
A number of studies have reported harmful effects from nanoparticles, e.g. they “can trigger cytotoxic, genotoxic, inflammatory and oxidative stress responses in mammalian cells”. This has led some researchers to caution against their use until they’re better understood, because there’s still a lot left to learn. One important issue that appears to need further investigation is the way proteins can stick to nanoparticles once they’ve entered the bloodstream, despite the addition of PEG.
“Absorption of nanoparticles is also possible when the nanomaterial first interacts with proteins or cells and might affect organs such as liver, brain, spleen, blood, kidney, heart, colon, bone, etc., and cause deleterious cytotoxic effects leading to deformation and inhibition of cell growth in humans and animals.”
“While linking RNA to molecules offers some level of protection against degradation, it can promote binding to serum proteins and subsequent aggregation that can lead to vascular blockage.”
Various physical and chemical properties of nanoparticles affect their toxic effects on cells and organs, such as their size and shape, what they’re made of, and how they’re made. For instance, particles less than 100 nm in size “can cause undesirable effects such as passing through the blood-brain barrier and triggering immune reactions as well as damaging cell membranes”.
Lipid interactions
Exosomes are quite similar to LNPs in that they are little lipid packages containing information, and they can affect the balance of lipids in a cell. They are excreted from cells, and are involved in trafficking information around the body. They are able to “fuse with the target cell membrane to send their own content into the cytosol, altering the physiological or pathological state of the recipient cell”. (This is also what viruses do.)
Once thought of as just “cellular dust”, exosomes are now known to be involved in lipid metabolism. They take bits of information from the cell, and transport it to other cells, e.g. lipids, proteins and RNA. Because of this, they can contain information about some illnesses which are linked to disorders of lipid metabolism, such as atherosclerosis, cancer, and Alzheimer’s. It’s also possible that microbes could set up camp in the cell, and make a bit of a mess – when a pathogen such as Hepatitis C, Chlamydia or Borrelia (Lyme Disease) causes chronic infection, it can disrupt various cellular processes, giving rise to non-specific symptoms like headache, brain fog, and aching joints. These persistent pathogens can create havoc inside cells, disrupting their metabolism, and the way they generate energy. They can also lead to the production of reactive oxygen species, which damage the cell membrane, so becomes like a leaky battery, and can’t function properly.
DR.GARTH NICHOLSON – WEAPONIZED MYCOPLASMA
Lipid Metabolism
The ratio of lipids in a cell changes according to the activity being performed, as well as according to individual physiology. For instance, changes in the surface tension of the lungs are critical for the regulation of breathing, and are dependent on the different proportions of neutral lipids relative to phospholipids. These proportions change according to the circumstances, and they can change quickly. For example, there’s cholesterol in the lining of the lungs (as part of the ‘surfactant’), and it changes according to what the lungs are doing at the time, e.g. doing exercise – trained athletes doing an intense activity tend to get a rapid decrease in cholesterol in lung surfactant, whereas not-so-fit people might get an increase.
Cholesterol affects various characteristics of cell membranes, such as how fluid they are, and how permeable they are, and it may regulate the function of proteins in the membrane. Lipids are also involved in helping cells signal information to other cells, whilst changes in lipids and cholesterol ratios can be related to certain afflictions:
“…the ratio between the amounts of cholesterol and lipids is important in the diagnosis of diseases such as Alzheimer’s, cancer, HIV, atherosclerosis, depression and many others. This balance of lipids and cholesterol is linked to the phase behaviour of lipids”
“… the complex molecular interactions between saturated lipids, unsaturated lipids and cholesterol give rise to a rich phase behaviour of biomembranes composed of these molecules.”
Cholesterol has been added to both the Moderna and Pfizer vaccines; it’s said to provide mobility to the other lipids in the LNPs.
Reactions to the swine flu vaccine (1976)
The swine flu vaccine used in 1976 was associated with a number of people developing a neurological disorder called Guillain-Barré syndrome (GBS). Some have suggested this was due to the production of anti-ganglioside antibodies. Gangliosides are a type of glycolipid (with sugary bits called glycans); they are found naturally in plasma membranes, but some of the glycans are similar to those found on bacteria called Campylobacter jejuni, so if people have this bacteria in their body, they can end up making antibodies that target both. This type of cross-reaction is what causes autoimmune disease, via molecular mimicry.
“Gangliosides … serve as surface receptors for bacterial toxins and, perhaps, normal, endogenous extracellular molecules. Also, these complex glycolipids have an influence on the electrical field across the cellular membrane, as well as the concentration of ions on the external surface of the cells. In addition, gangliosides may have a role in electrical insulation in myelin cells in the nervous system.”
Lipids are also involved in the development of allergic responses, and “among major allergens there is a high frequency of lipid-binding proteins.” An example of this is Lipid Transfer Protein Syndrome, which is a sensitivity to some kinds of lipids in plant-based foods, and can cause anaphylaxis.
Lipid adjuvants – what’s changed?
Old Version (unprocessed lipids and real bacteria): for many years, vaccines were formulated with the Complete Freund’s Adjuvant (CFA), which is a water-in-oil emulsion. Used since 1937, CFA contained paraffin oil and killed mycobacteria, but was eventually replaced with a less dangerous version (i.e. without mycobacteria). An extreme example of what the adjuvant could do to a person occurred in 1975, when a scientist accidentally injected some of it into his finger. His finger began to swell, and he got bad chest pain, fever and the shakes. He continued to get very ill with a range of problems, and tests showed he was producing anti-phospholipid antibodies, which usually indicates an autoimmune disorder. He also kept getting Epstein Barr infections, and suffered from “extreme fatigue, sleep disturbances, depression, orthostatic hypotension, falls, and fainting. Each of these episodes lasted for 8 to 10 months”. He also experienced a lot of problems with his heart (congestive heart failure).
Sometimes called ‘M. tuberculosis death cells’, mycobacteria can cause chronic (long-term) infections, and CFA is used in experiments on mice to induce a disease that’s similar to multiple sclerosis.
New Version (complex lipid nanoparticles and fake bacteria): LNPs can be used to deliver a variety of genetic or molecular payloads, including adjuvants. Some lipids can also be formulated to ‘look like’ bacteria. In the form of nanoparticles, the new synthetic lipids can reach places the old lipids couldn’t – right inside the cell. They’re as small as natural biomolecules, so they can “reach intra-cellular structures that were previously accessible only to biological aggressors.”
By engineering lipid adjuvants at the nano-scale, it’s possible to exploit the immune system by tricking it into believing a real pathogen has arrived, which can then trigger the same immune reaction that a real pathogen would. Special cells called ‘toll like receptors’ (TLRs) can recognise various genetic and molecular parts of pathogens, such as DNA, RNA, lipids, and sugary molecules called saccharides. They will then mount an immune response tailored to suit that pathogen, like the anti-ganglioside antibodies described above.
A widely known example of adverse reactions to LNPs happened in 2009, the last time we had a fake pandemic. After the WHO declared swine flu to be a threat, millions of people received a vaccine, one of which was called Pandemrix. This vaccine is linked to hundreds of people developing a debilitating ‘sleeping sickness’ called narcolepsy; some of them also developed cataplexy, which is characterized by a sudden loss of muscle tone. What makes this event especially significant is that they all had two things in common: 1) a specific type of narcolepsy, where there’s a lack of hypocretin in the central nervous system, and, 2) a particular gene (DQB1*06:02).
Pandemrix contained recombinant proteins from the swine flu virus, and an adjuvant called ‘AS03’. Described as an ‘oil-in-water’ adjuvant, AS03 is being used in the coronavirus vaccine made by Sanofi/GSK. It contains a lipid called squalene, which has been linked to Gulf War Sydrome, as well as DL-α-tocopherol, which is a synthetic version of Vitamin E, but with a different structure and composition to the natural form. Vitamin E is soluble in lipids, and is linked to phospholipid transfer proteins. AS03 also contains polysorbate 80 as a surfactant (i.e. to maintain the surface tension of the nanoparticles), so it’s difficult to untangle what does what.
A team of researchers from Stanford, investigating possible causes of the narcolepsy cases, suggested a link between parts of the flu virus, and the hypocretin gene. Hypocretin plays a significant role in the regulation of basic physiological processes such as the sleep cycle, and “narcolepsy with cataplexy is caused by hypocretin deficiency”. They found similarities between hypocretin proteins produced in the body, and some of the proteins produced by the virus, which could make people develop antibodies that attack both the flu proteins, and their own hypocretin proteins. The flu proteins could come from either the vaccine, or from natural infection, but having the gene is what made it happen in only one group of people.
(It’s interesting to note here that adding proteins and peptides to nanoparticles during production can actually change their structure; for instance, the fusion peptide from influenza can make a lipid bilayer change to a non-bilayer cubic phase, and so-called ‘cubosomes’ have different properties to other lipid structures. Perhaps this potential is controlled during production of LNPs, but is it possible that proteins from a natural infection with influenza could also change the nature of the lipid bilayers once inside the body? What studies have been done to investigate changes to LNPs in various physiological environments?)
More recent research links loss of hypocretin to the vitamin E in AS03, saying that people with a genetic predisposition (i.e. having the DQB1*602 gene) could be triggered by the vitamin E, and then develop narcolepsy due to the activation of a gene called ‘NRF2’, along with signals from dying cells. NRF2 helps with the metabolism of cells, but some kinds of lipids can suppress this function.
Aside from all this, lots of other studies have been done on AS03, and the precise effects it has on immune reactions. One study reported that it changed the balance of lipids in parts of the cell;
“…AS03 affects cholesterol and fatty acid metabolism of macrophage cells in lymph nodes, showing a decrease of cholesterol but an increase of phosphatidylcholine lipids, a class of phospholipids. The alteration of the cellular phospholipid composition leads to disorder of one particular compartment of the cell: the endoplasmic reticulum (ER). This is known as ER stress.”
This, however, is perceived as being a good thing because it’s an adjuvant effect, and the immune response associated with ER stress is desirable for some vaccines. But is it wise to mess with the ER in this way? The endoplasmic reticulum is the major “lipid factory” within the cell, whilst “emerging evidence suggests ER stress…. participates in various pathologies including neurodegeneration, inflammation, metabolic disorders, and infectious diseases”. (By the way, the ER is also involved in processing antigens, such as those produced by the mRNA sequences in the Moderna and Pfizer vacccines.)
Inducing ER stress is just one example of what is being called ‘precision vaccinology’. It’s about targeting specific cells in the body, to produce a particular kind of reaction. Another example of this is how AS03 can activate genes called MX1 and STAT1, and induce the production of neutrophils, etc. (Neutrophils are a type of white blood, and they tend to increase in response to infection, stress or injury.) It can also lead to “the upregulation of CD4 T cell responses and IFNγ release”.
AS03 is part of a range of adjuvants developed by GlaxoSmithKline – the other three (AS01/02/04) contain monophosphoryl lipid A (MPLA) from the lipopolysaccharides in the cell wall of Salmonella bacteria; acting as a molecular mimic, the lipid A portion can stimulate TLR4, just like real Salmonella.
Okay, that’ll have to do for now… if you’ve read this far, then hopefully now you’ll appreciate that LNPs are more than ‘just a bit of oil’, and yet they’re not microchips designed to control you (even if they could fit through a needle, they could end up almost anywhere). They are the result of ten years of experimentation with mRNA vaccines, including human trials. Moderna and the NIH have done a lot of the legwork, and have collaborated on some projects, but no-one seems to be citing these trials as ‘evidence of safety’….
Image credit: Wyatt Technology
Become a Patron!
Or support us at SubscribeStar
Donate cryptocurrency HERE
Subscribe to Activist Post for truth, peace, and freedom news. Follow us on Telegram, SoMee, HIVE, Flote, Minds, MeWe, Twitter, Gab and Ruqqus.
Provide, Protect and Profit from what’s coming! Get a free issue of Counter Markets today.

Be the first to comment on "Ronavax Roulette: Issues with Lipid Nanoparticles (Part One)"