Jeff Berwick
Activist Post
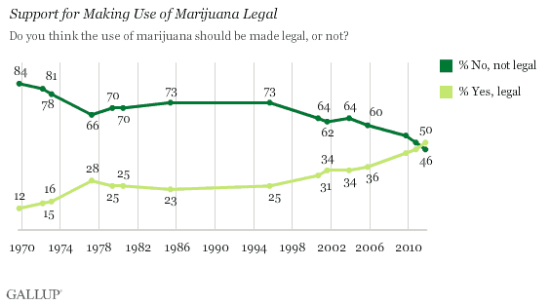
Like with most things, the government sits well on the side of tyranny compared to the general public when it comes to marijuana policy. A record number of Americans believe marijuana should be legal:
The government is slowly shifting its policy on marijuana, evidenced by Washington and Colorado legalizing it for recreational use as well as the many states legalizing it for medicinal use. The federal government, historically content locking up individuals for victimless marijuana crimes and throwing away the key, might now be liberalizing its view on marijuana.
The Food and Drug Administration (FDA) is reportedly analyzing whether or not the US should downgrade the classification of marijuana as a Schedule 1 drug at the request of the Drug Enforcement Administration (DEA), which would be a sea change in policy and could potentially change the makeup of the American prison system much to the chagrin of the prison-industrial complex.
The FDA is well-aware that over the last few decades there has been increased interest in the potential utility of marijuana for numerous medical conditions, “including those that already have FDA-approved therapies.” The FDA Continues:
More recently, several states have also passed laws that remove state restrictions on health care professionals using marijuana as a medical treatment for a variety of conditions. A number of other states are considering similar legislation regarding the use of marijuana in medical settings.
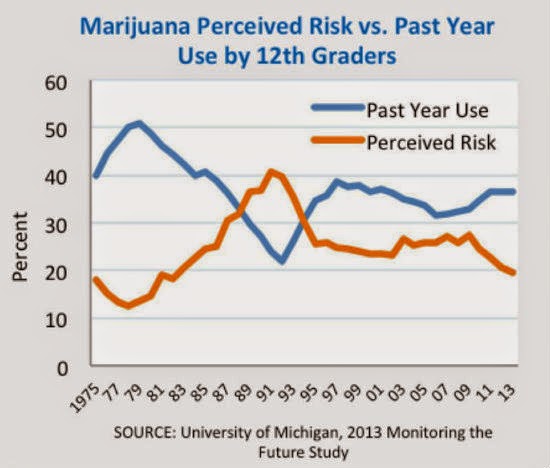
The global marijuana legalization movement shows no signs of slowing down with both New York and Florida recently setting the groundwork for the future decriminalization of marijuana. High schoolers in the US are smoking more marijuana as the perceived danger of marijuana drops:
Despite overwhelming evidence the FDA still maintains a rather atavistic disposition on marijuana, stating on its website: “The FDA has not approved marijuana as a safe and effective drug for any indication.”
In spite of this the agency has approved one drug in which a synthetic version of a substance found in marijuana is present and another drug, which features a synthetic substance that acts similarly to compounds found in cannabis but is not present in cannabis. On its website the FDA hints at an evolving cannabis policy:
Although the FDA has not approved any drug product containing or derived from botanical marijuana, the FDA is aware that there is considerable interest in its use to attempt to treat a number of medical conditions, including, for example, glaucoma, AIDS wasting syndrome, neuropathic pain, cancer, multiple sclerosis, chemotherapy-induced nausea, and certain seizure disorders.
This is great not only for the economy but also for the health and wellbeing (and freedom) of millions of people in the US. As I am sure even the FDA has to admit, there is an immense amount of good which could come from this single herb, cannabis. It is, after all, about time as the DEA has been trying to get the FDA to re-classify the drug since 2001! Is there any hope they will reschedule marijuana soon? On its website, the FDA spells out what must be done:
 “Before conducting testing in humans of a drug that has not been approved by the FDA, an investigator submits an investigational new drug (IND) application, which is reviewed by the FDA. An IND includes protocols describing proposed studies, the qualifications of the investigators who will conduct the clinical studies, and assurances of informed consent and protection of the rights, safety, and welfare of the human subjects. The FDA reviews the IND to ensure that the proposed studies, generally referred to as clinical trials, do not place human subjects at unreasonable risk of harm. The FDA also verifies that there are adequate assurances of informed consent and human subject protection.”
“Before conducting testing in humans of a drug that has not been approved by the FDA, an investigator submits an investigational new drug (IND) application, which is reviewed by the FDA. An IND includes protocols describing proposed studies, the qualifications of the investigators who will conduct the clinical studies, and assurances of informed consent and protection of the rights, safety, and welfare of the human subjects. The FDA reviews the IND to ensure that the proposed studies, generally referred to as clinical trials, do not place human subjects at unreasonable risk of harm. The FDA also verifies that there are adequate assurances of informed consent and human subject protection.”
This process seems to be underway.
“FDA Supports Sound Scientific Research” – FDA
The FDA is integral in deciding how far scientific research into the medical uses of marijuana goes. The agency has come out and said, “As a part of this role, the FDA supports those in the medical research community who intend to study marijuana.” The FDA goes on:
The FDA also supports research into the medical use of marijuana and its constituents through cooperation with other federal agencies involved in marijuana research…
…Several states have either passed laws that remove state restrictions on the medical use of marijuana and its derivatives or are considering doing so. The FDA supports researchers who conduct adequate and well-controlled clinical trials which may lead to the development of safe and effective marijuana products to treat medical conditions. We have talked to several states, including Florida, Georgia, Louisiana, New York and Pennsylvania, who are considering support for medical research of marijuana and its derivatives to ensure that their plans meet federal requirements and scientific standards.
That US Regulators are open to easing restrictions on marijuana is certainly a step towards federal decriminalization, which places the US – along with a few other jurisdictions – at the forefront of the global marijuana legalization.
The reversal is indeed huge with major implications for the cannabis industry, as marijuana is currently a Schedule 1 drug. This means harmless cannabis carries the most amount of restrictions possible. Schedule 1 drugs are considered to have no medical benefit and are highly addictive, both of which have been proven patently false in scientific studies for cannabis.
The FDA reviewed marijuana’s status for the DEA in 2001 and 2006 and recommended both times to keep it Schedule 1. The DEA has since been petitioned to change marijuana’s classification.
Politicians from both side of the aisle protect medicinal marijuana. They don’t have much of a choice as being against marijuana legalization will cost any politician in nearly any state throughout the US the young vote.
As we cover in the TDV Newsletter the “War on Drugs” is coming to a close and this will create massive opportunities for profit. The TDV Golden Trader will be covering the entire medical marijuana industry and the opportunities for profit in that sector.
Anarcho-Capitalist. Libertarian. Freedom fighter against mankind’s two biggest enemies, the State and the Central Banks. Jeff Berwick is the founder of The Dollar Vigilante, CEO of TDV Media & Services and host of the popular video podcast, Anarchast. Jeff is a prominent speaker at many of the world’s freedom, investment and gold conferences as well as regularly in the media including CNBC, CNN and Fox Business


Be the first to comment on "Is The FDA Laying The Groundwork For Resecheduling Marijuana?"